Understanding Cation Selectivity in Carbon Nanopores with Hybrid First-Principles/Continuum Simulations: Implications for Water Desalination and Separation Technologies
 ACS Appl. Nano Mater. 2020, 3, 10, 9740–9748
ACS Appl. Nano Mater. 2020, 3, 10, 9740–9748
Cheng Zhan, Fikret Aydin, Eric Schwegler, Aleksandr Noy, and Tuan Anh Pham
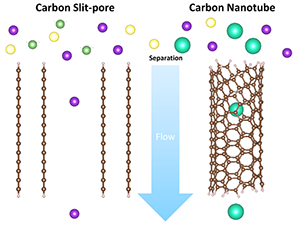
The abstract reads as follows: Understanding ion adsorption in porous carbons is critical for a wide range of technologies, including water desalination and energy storage. In this work, we combined density functional theory with a continuum solvation model to investigate thermodynamics and kinetics of the adsorption process of alkali metal ions from aqueous solutions into carbon nanopores with different sizes and geometries. We found that cations with a larger ionic radius are more favorable to enter the nanopores because of a lower energy penalty of dehydration. In addition, the pore size and geometry were found to have a significant impact on the ion–pore interaction under confinement and cation selectivity. Our study highlights a complex interplay among nanopore geometry, ion size, and hydration on the cation adsorption selectivity, suggesting that tuning the porosity could represent a general strategy for improving ion separations.
All the authors were supported as part of the Center for Enhanced Nanofluidic Transport, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award no. DE-SC0019112.
https://pubs.acs.org/doi/10.1021/acsanm.0c01842
