Modulation of Charge Density and Charge Polarity of Nanopore Wall by Salt Gradient and Voltage
ACS Nano 2019, 13, 9, 9868-9879
Chih-Yuan Lin, Elif Turker Acar, Jake W. Polster, Kabin Lin, Jyh-Ping Hsu and Zuzanna S. Siwy.
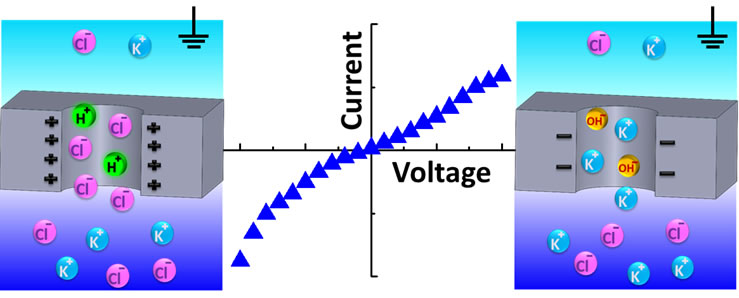
The publication directly addresses KG5 and KG6. The experimental and modeling work showed that surface charge of nanopores placed in contact with salt gradient is not a constant value but rather depends on the magnitude of salt gradient and voltage. It was found that even when a nanopore was in contact with solutions of pH equivalent to the isoelectric point of the pore surface, the pore walls became charged with voltage-dependent charge density. Thus a nanopore that is uncharged at 0 V at any salt conditions, when placed in a salt gradient can become positively charged at one voltage polarity and negatively charged at the opposite polarity. Experiments were performed with single 10 nm in diameter nanopores prepared by the process of dielectric breakdown.
The abstract reads as follows: Surface charge plays a very important role in biological processes including ionic and molecular transport across a cell membrane. Placement of charges and charge patterns on walls of polymer and solid-state nanopores allowed preparation of ion-selective systems as well as ionic diodes and transistors to be applied in building biological sensors and ionic circuits. In this article, we show that the surface charge of a 10 nm diameter silicon nitride nanopore placed in contact with a salt gradient is not a constant value, but rather it depends on applied voltage and magnitude of the salt gradient. We found that even when a nanopore was in contact with solutions of pH equivalent to the isoelectric point of the pore surface, the pore walls became charged with voltage-dependent charge density. Implications of the charge gating for detection of proteins passing through a nanopore were considered, as well. Experiments performed with single 30 nm long silicon nitride nanopores were described by continuum modeling, which took into account the surface reactions on the nanopore walls and local modulation of the solution pH in the pore and at the pore entrances. The results revealed that manipulation of surface charge can occur without changing pH of the background electrolyte, which is especially important for applications where maintaining pH at a constant and physiological level is necessary. The system presented also offers a possibility to modulate polarity and magnitude of surface charges in a two-electrode setup, which previously was accomplished in more complex multielectrode systems.
Research was supported as part of the Center for Enhanced Nanofluidic Transport, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), under Award No. DESC0019112 (development of the model and data analysis, preparation of nanopores by dielectric breakdown and transmission electron microscopy).
