Theory of Surface Forces in Multivalent Electrolytes
 Langmuir 2019, 35, 11550−11565
Langmuir 2019, 35, 11550−11565
Rahul Prasanna Misra, Pedro de Souza, Daniel Blankschtein, and Martin Z. Bazant
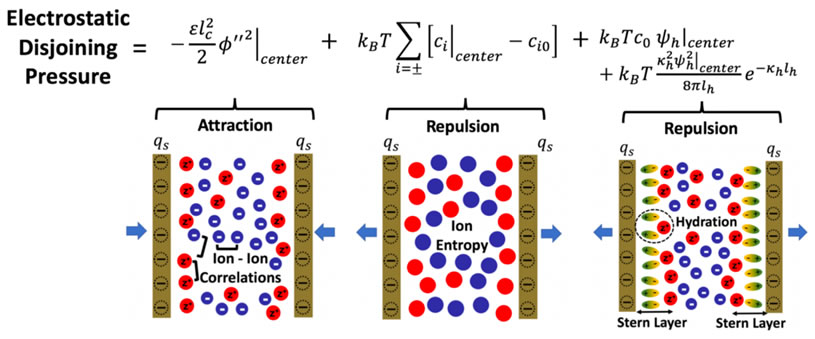
The abstract reads as follows: Aqueous electrolyte solutions containing multivalent ions exhibit various intriguing properties, including attraction between like-charged colloidal particles, which results from strong ion−ion correlations. In contrast, the classical Derjaguin−Landau−Verwey−Overbeek theory of colloidal stability, based on the Poisson−Boltzmann mean-field theory, always predicts a repulsive electrostatic contribution to the disjoining pressure. Here, we formulate a general theory of surface forces, which predicts that the contribution to the disjoining pressure resulting from ion−ion correlations is always attractive and can readily dominate over entropic-induced repulsions for solutions containing multivalent ions, leading to the phenomenon of like-charge attraction. Ion-specific short-range hydration interactions, as well as surface charge regulation, are shown to play an important role at smaller separation distances but do not fundamentally change these trends. The theory is able to predict the experimentally observed strong cohesive forces reported in cement pastes, which result from strong ion−ion correlations involving the divalent calcium ion.
R.P.M., J.P.d.S., D.B., and M.Z.B. acknowledge the financial support received as part of the Center for Enhanced Nanofluidic Transport (CENT), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award # DE-SC0019112, for formulating the theory of surface forces and the accompanying analysis presented in this paper.
https://pubs.acs.org/doi/10.1021/acs.langmuir.9b01110
